Chlorobenzene: Synthesis, applications and safety hazards
Chlorobenzene is an aromatic organic compound which has the chemical formula C6H5Cl. It is a flammable liquid that has a clear appearance and smells like almonds to some extent. This is the most elementary form of the class of monochlorobenzenes, which encompasses all benzenes in which a single hydrogen has been replaced by chlorine. It is used as a solvent and its chemical structure is as following:
Chlorobenzene is a liquid that is colorless to slightly yellowish and has the aroma of delectable almonds. The temperature at which an explosion can occur is 84 degrees Fahrenheit. It is almost entirely insoluble in water and has a density of 9.2 pounds per gallon, making it considerably denser than water. It will start to melt at -49 degrees Fahrenheit and will start to boil at 270 degrees Fahrenheit when the pressure is 760 millimeters of mercury. It is also known as CHLOROBENZENE, 108-90-7, monochlorobenzenes, phenyl chloride, and benzene chloride, amongst a number of other names.

At lower concentrations, it is possible for it to dissolve in water. This is despite the fact that it readily evaporates into the air. It is not at all found in nature and cannot be discovered in its natural state. The production of chlorobenzene in the United States has decreased by more than 60 percent since the 1960s, when it was at its highest point. This was the beginning of the creation of a great number of additional compounds, such as phenol and DDT. These days, chlorobenzene is used for a wide number of purposes, including acting as a chemical intermediate in the manufacturing of other chemicals, serving as a degreasing agent for automobile components, and playing the role of a solvent in the creation of some pesticide formulations.
Synthesis
It was given its official name in 1851. It is necessary to have a catalytic amount of a Lewis acid present in order to produce chlorobenzene. Some examples of Lewis acids are ferric chloride, Sulphur dichloride, and anhydrous aluminum chloride.
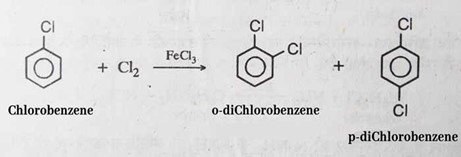
Because of the catalyst, the chlorine takes on an electrophilic character. Due to the electronegative nature of chlorine, C6H5Cl is somewhat less susceptible to additional chlorination than C6H5. In order to generate the smallest amount of dichlorobenzene possible, this reaction is typically carried out in a continuous fashion in commercial and manufacturing settings.
These days, the vast majority of monochlorobenzene is created in plants that are operational around the clock from benzene and chlorine. There are two potential outcomes, both of which are determined by the ratio of benzene to chlorine: either a low rate of benzene conversion and minimal dichlorobenzene formation, or an almost complete rate of benzene conversion with a greater degree of dichlorobenzene creation. Both of these outcomes are dependent on the benzene-to-chlorine ratio. It has been reported that a chlorination combination that achieves the highest possible concentration of monochlorobenzene consists of 4%–5% unreacted benzene, 73% monochlorobenzene, and 22–23% dichlorobenzene. This combination is able to achieve this concentration because it contains 73% monochlorobenzene.
Uses
Chlorobenzene has the following uses:
- In the manufacture of pesticides, the production of diisocyanate, the degreasing of automobile components, and the synthesis of nitrochlorobenzene, the use of chlorobenzene as a solvent is by far the most common application.
- At one point in time, chlorobenzene was an essential component in the production of both phenol and the insecticide DDT.
- It is used as a dry cleaning agent and as an intermediary in the manufacture of organic compounds; a solvent for paints, adhesives, polishes, waxes, and natural rubber. Also used as an intermediate in the production of organic compounds. In the textile industry, it is used as a solvent for bitumen and asphalt coatings on buildings, as well as a medium for the transfer of heat, an agent for swelling fibres, and a dye carrier.
- On occasion, businesses in the dry-cleaning sector take use of this compound.
Hazards
- Due to the fact that the LD50 value for chlorobenzene is so low (just 2.9 grams per kilogram) its level of toxicity is classified as "low to moderate." Over the course of an eight-hour time-weighted average, OSHA permits personnel in industries that use chlorobenzene to be exposed to a maximum of 75 parts per million (350 milligrams per cubic meter) of the chemical.
- Animals that were given chlorobenzene experienced lethargy, tremors, and spasms in their muscles after being exposed to the chemical. Chronic exposure to chlorobenzene, also known as long-term exposure, has been shown to have detrimental effects on the human central nervous system (CNS). A loss of sensation, cyanosis, hyperesthesia (increased sensitivity), and muscle spasms are some of the signs of neurotoxicity in humans.
- On the other hand, there is no information on chlorobenzene's potential to cause cancer in people. The EPA has placed chlorobenzene in Group D, which indicates that it is not possible to determine with sufficient certainty whether or not it is carcinogenic to people.